
Experiment Overview:
Thanks to solar panels, the sun’s power is harnessed for clean energy production through photovoltaic technology. The sun also has incredible thermal power. Solar heat, or solar thermal energy, is a form of energy that uses the sun’s power to heat items. Heat results when light energy from the sun strikes atoms. As the atoms become activated by the heat, thermal energy is produced. Let’s break this down a bit further.
All objects in our universe are made up of matter and energy. If we look at matter with a magnified lens, we will see that all matter is made up of tiny molecules (groups of atoms). In cold items, the molecules move very slowly and have a limited amount of energy. When you begin to heat an item, the molecules speed up; the faster they move, the hotter the object becomes. The sun’s light can actually cause this molecular reaction, and create environmentally-friendly thermal energy.
In this experiment, we are going to passively collect the sun’s thermal energy and heat up matter. So, let’s get this experiment started and get those molecules moving!
Experiment Materials:
- 6 empty soda pop cans
- Paint (6 different colors)
- 1 Paint brush
- Outside thermometer or an app/website that provides local weather
- Cooking thermometer
- Here is an example of what you will need- Click for link
- Water
- 1 Funnel
- 1 Bucket
- 1 Measuring cup
- Data collection notebook (any notebook will work or a sheet of paper)
Experiment Process:

Step 1
The first step is to prepare the 6 soda pop cans for the experiment. Empty the cans of their original fluids, clean them out, and then let them dry.

Step 2
Next, paint the cans different colors. Make sure to paint the entire can (even the top) so that none of the original markings are showing. Also, make sure to select a variety of colors from dark to light. The paint colors we recommend are as follows: black, white, green, purple, dark blue and light blue/teal. Note: you may need to put on multiple coats of paint.

Step 3
Fill the cans with water. To make sure all of the cans start at the same temperature, fill a bucket with water and take your water supply from the bucket so that it is consistent. Fill each can with 3/4 cup of water with the help of a funnel. Before you discard your bucket of water, take a temperature reading so that you have a base temperature for the water. We recommend starting with a cooler water temperature.

Step 4
The next step is to place the cans in a sunny place to harness the most thermal energy. Leave the cans outside over the span of a day, at least six hours. If the cans ever end up in the shade as the day progresses, move them to a place with full exposure to the sun.

Step 5
Over the course of the day, chart the temperature change of the water in each pop can using a cooking thermometer. Each hour, around the same time, use the thermometer to chart the temperature of each pop can and the temperature outside.
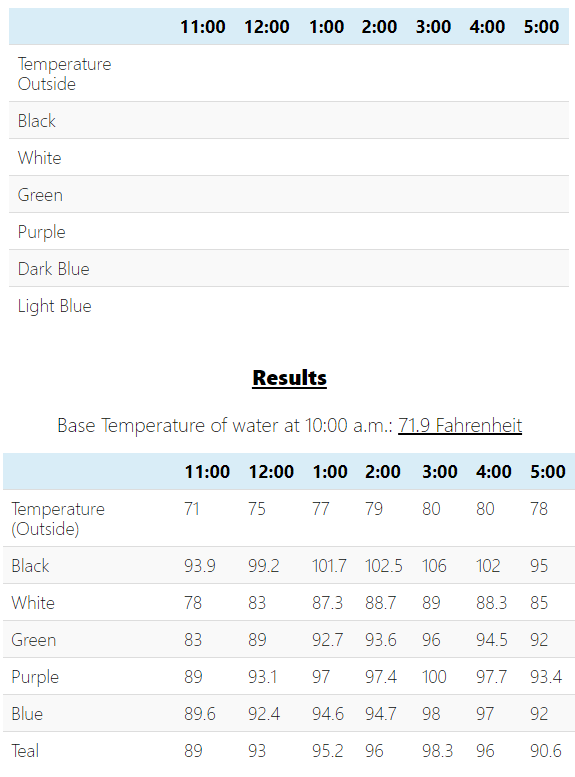
You can use the below table so to easily organize the data and see the temperatures throughout the day.
Base Temperature of water at 10:00 a.m:__________
Conclusions:
Take Away 1: The first thing to note in this experiment is the correlation between the outside temperature and the water inside the cans. At the hottest point of the day, the water in the pop cans will likely have also reached their highest temperature. In this example, the temperature peaked around 3:00 pm (for both the outside temperature and the water temperature, and slowly decreased as the sun moved to a lower location in the sky. This happened because at the hottest part of the day, the cans were able to capture the most solar thermal energy!
More, or longer, exposure to sunlight and hotter temperatures equals a higher water temperature.
Take Away 2: It is important to state that dark-colored objects absorb all wavelengths of light and therefore convert more of the sun’s energy into heat. The more sunlight an object absorbs, the more heat! Colors like white have the opposite effect and absorb limited light energy, which creates a smaller amount of thermal heat. With this information, it is easy to see why the black and dark purple pop cans absorbed the most thermal energy while the white and green produced the smallest increase in temperature.
The darker the color, the more light/thermal energy absorbed. Light colors have the opposite effect.
Explanation
To better understand what was accomplished in this experiment, we need to discuss solar water heaters. There are two basic types of solar water heaters – active and passive. In this experiment, we created a passive solar water heating system. The system is “passive” because it did not use any mechanical parts to aid in the heating process or circulation of water. An active system relies on pumps and other working parts to circulate and distribute the heated water. No matter if you create an active or passive heating system, you are utilizing the sun’s light as a means to heat an object.
It’s important to note that thermal heating does not always need water. In this experiment, water was used as a way to better understand how the sun’s light can heat an object. The sun’s renewable energy can be used in many different ways to create solar heat both with and without the use of water.
- Passive solar heating examples: heating a greenhouse with the sun’s rays to produce a food source, south facing windows in a home to collect heat, building materials that absorb heat to cut down on energy costs, a sunroom in a home to take advantage of the sun’s heat, thick stone floors and insulated walls to keep energy in a building
- Active solar heating examples: solar collectors positioned on a roof to heat water which then pumps the water through an entire building to use as a heat source, a solar thermal heating system for a pool, an air solar system that uses fans, controls, and ductwork to heat air in a home
Study/Extension Questions
- What is the difference between passive and active heating systems?
- What is the impact of materials and colors on capturing thermal power?
- What are examples of passive and active solar heating found around your home/neighborhood?
Extension:
Variations to Experiments
- Try using a different object as your water collection item (plastic containers, glass jars) and ask the question: Does the container make a difference when dealing with the results?
- Try painting the cans different colors and evaluate the impact.









